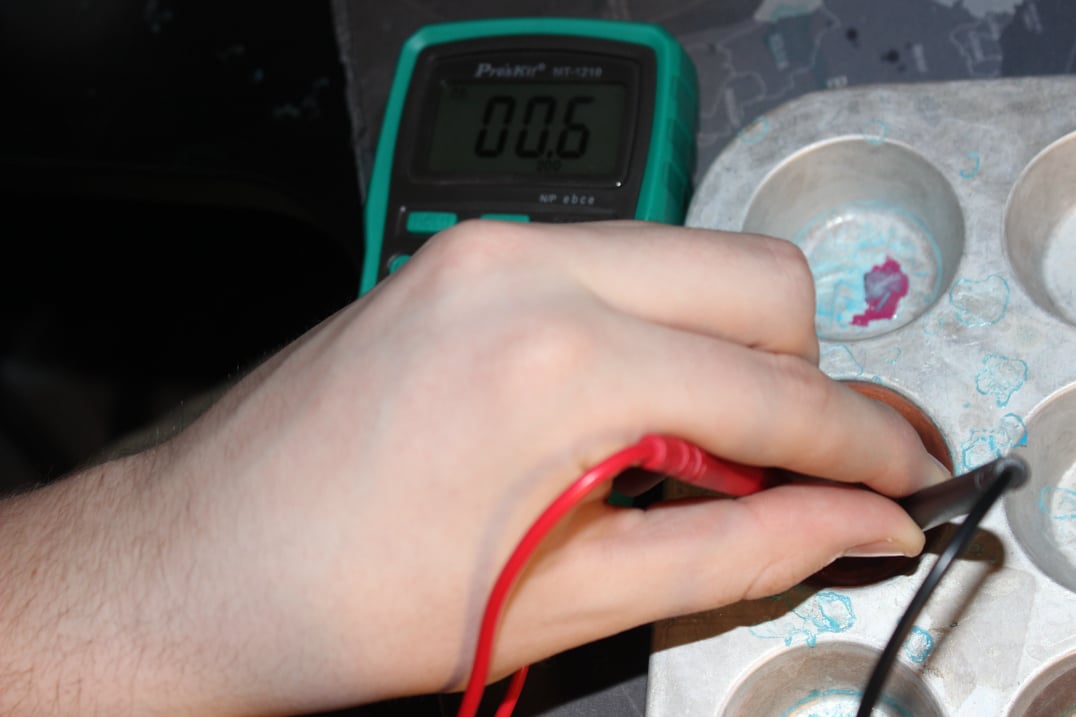
Materials
The materials are very similar to part one except instead of normal water we have a correctly mixed solution of Copper (II) Sulfate. Another notable change was that of the source of copper, instead of the original copper pipe I bought some high purity copper sticks to ensure a better reaction.